
A tale of transgenic mice: Heart failure medication might prevent, and reverse Alzheimer’s Disease
By Patricia Sharleen, March 3 2021—
How do we treat Alzheimer’s Disease (AD)? Almost every professional that has worked closely with dementia patients knows that AD is progressive and fatal. But what if it isn’t? What if there was a drug that could prevent or even treat it?
As of today, AD is a progressive neurodegenerative disorder, the most common type of dementia that in advanced stages, causes severe memory and learning impairments as well as loss of motor function. One of the hallmarks of a brain affected by AD is the presence and accumulation of a sticky protein called beta-amyloid (Aβ), or Aβ plaques between nerve cells. This accumulation and deposition is thought to disrupt cell-to-cell communication and ultimately result in neuronal cell death and decreased brain volume. It is believed to be the primary driving force of AD pathogenesis. This idea, which is referred to as the “amyloid hypothesis,” has been the mainstream concept used to explain AD progression for the past two decades. Even then, it is crucial to note that not all researchers are convinced that beta-amyloid is the primary cause of Alzheimer’s, but most agree that it is a key component to understanding the disease.
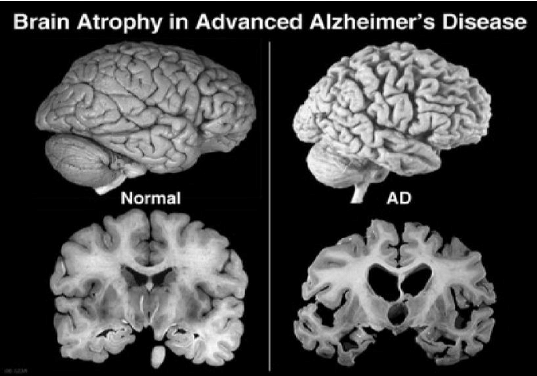
Importantly, an increasing amount of evidence has been gathered that shows that neuronal hyperactivity — an increased activity in the brain in the form of aberrant electrical impulse caused by synaptic dysfunction — is a symptom of early-stage AD that can be observed even before the formation of Aβ plaques. The efficient internal functioning of the brain depends on meaningful and structured transmission of neural impulses. This heightened responsiveness of neural networks related to the lowered thresholds of depolarization can obscure meaningful signals and can result in obstruction of basic cognitive functions such as thinking and memory recall as well as sensory inputs and peripheral reflex responses. In addition, recent studies have shown that hyperactivity can promote the production of Aβ and that brain areas that are more active are more prone to Aβ plaque deposition, creating a vicious cycle. However, at this moment, the underlying mechanism is still not well understood.
One approach taken to break the cycle is to target the Aβ cascade and reduce Aβ depositions. This method has proven to be insufficient in the past, and no Aβ-targeting drug candidate has been successful in halting this neurodegenerative process. This lack of Aβ-focused treatment has researchers focusing on its evil twin sister, neuronal hyperactivity.
At the University of Calgary, Dr. S.R. Wayne Chen and his team have also dedicated their time in researching and developing novel therapeutics for AD. Chen’s research focuses on Ryanodine receptor 2 (RyR2), an intracellular Ca2+ release channel found in the heart and brain that is responsible for controlling membrane excitability and AD-related neuronal hyperactivity. Dr. Wayne Chen, PhD, a professor in the department of Physiology & Pharmacology.
Their most recent findings published by Cell Reports in the paper “Limiting RyR2 Open Time Prevents Alzheimer’s Disease-Related Neuronal Hyperactivity and Memory Loss but Not β-Amyloid Accumulation” has demonstrated the possibility of R-carvedilol in preventing memory loss in mice that have not exhibited AD symptoms and rescuing memory deficits in mice with late stage AD.
The study involved cross-breeding 5xFAD transgenic mice, mice that are genetically engineered with five AD-linked mutations resulting in early-onset AD pathology, with RyR2-E4872Q mouse model that has mutated residue responsible for shortening length of RyR2 open time.
“Previously, we have found that when the activity of RyR2 is inhibited, it can reduce the activity of the heart muscle in patients with heart disease. We were wondering if the same manipulation of RyR2 will also be able to reduce the activity of the neurons, so we crossed 5xFAD mouse model with the mutant [E4872Q] mouse model and saw that indeed, genetically inhibiting the RyR2 prevents the hyperactive neurons in the brain of the mice.” Remarked Dr. JinJing Yao, PhD, and first author of the paper.
R-carvedilol, a component of an existing heart-failure medication, was administered to different ages of 5xFAD mice, before and after the appearance of AD symptoms. “We have a potential drug which can work similarly to the mutant which also limits the activity of the receptor and we found that it calms hyperactive neurons and improves memory in the AD mouse model. Previously the memory of these mice were so bad they basically couldn’t remember anything.”
Just as importantly, this is done with continued β-amyloid accumulation. This begs the question, how can this be achieved while allowing for accumulation of something believed to be toxic to neurons?
Yao further explained that “To make it clear, we did see neuronal cell death in the aged 5xFAD mice. However, when we introduced the RyR2-E4872Q mutation into the 5xFAD mice, or treated the 5xFAD mice with R-carvedilol to short the RyR2 open time, the neuronal cell death seemed to be reversed. My understanding is, the neurons are not dying from Aβ directly, they are dying from the consequence of Aβ induced hyperactivity.”
Yao is hopeful that the study will progress into its clinical trial stage even though much further testing still needs to be done.
